Preclinical Imaging: Animals, Plants and Materials Imaging
NIF provides access to advanced in vivo micro-CT, in vivo multispectral fluorescence and bioluminescence imaging, in vivo high frequency ultrasound and photoacoustic imaging, large field of view CT and the 9.4T preclinical magnetic resonance imaging (MRI) instrument.
About our preclinical imaging systems
These systems can image anaesthetised small animals such as mice and rats non-invasively with real time physiological monitoring including respiration, animal temperature and heart rate. Animals can be imaged in real time and cells, protein or bacteria tracked over time, to provide a more biologically relevant understanding of tumour growth, disease progression or mechanisms of drug action.
Applications include the study of infectious diseases, oncology, cardiology, molecular biology, neurobiology, musculoskeletal, vascular, respiratory, inflammation, toxicology, metabolism, embryology, animal development, endocrine disruption, drug development and spectroscopy.
WA NIF also provides access to a Large Field-of-View X-Ray CT scanner dedicated for materials imaging and research. Applications range from biomedical, geological, marine science research to archaeology, fossil study and inspection of additively manufactured and other industrial components.
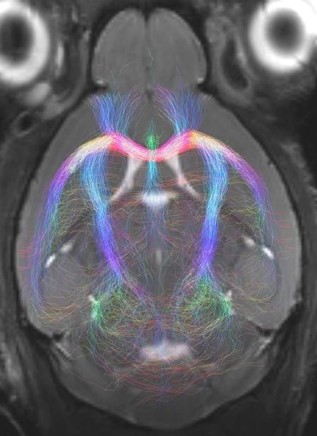
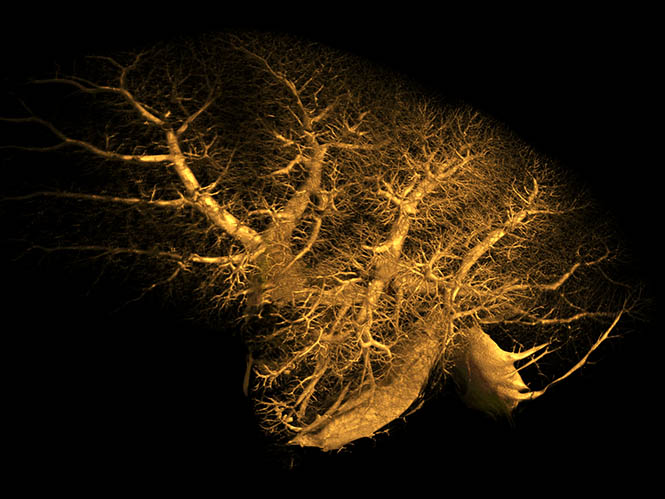
9.4T Magnetic Resonance Imaging (MRI)
Magnetic resonance imaging (MRI) uses applied magnetic fields to non-invasively (and non-destructively) image samples with non-ionising radiation (unlike X-ray based techniques). MRI can rapidly provide excellent in-vivo soft-tissue image contrast for qualitative analyses, 3D images for quantitative volumetric measurements, and access to parameter maps (for example, MR relaxation, diffusion, flow) related to underlying tissue structure. Rapid imaging techniques can also be used to study dynamic processes, such as the cardiac cycle.
A wide range of MRI experiments are available to study organ function, including blood oxygenation level dependent (BOLD) contrast (commonly used for functional MRI), perfusion, and vascular imaging. In addition, magnetic resonance spectroscopy (MRS) enables in-vivo NMR spectroscopy measurements to be made from predefined volumes of interest and facilitates the linkage of underlying biochemical processes to disease progression, treatment and the like.
MRI studies on nuclei other than protons (H-1) are also possible, including F-19, Na-23, and P-31.

Large Field of View Computed Tomography (CT)
Western Australia’s only Large Field-of-View (250mm x 250mm x 330mm), materials’ research dedicated CT, for non-destructive imaging of internal parts, using multiple axial scans to generate 2D cross-sectional information or 3-dimenional reconstructions. The X-ray CT has the typical mechanism for taking ‘2D X-Ray projections’ which are then digitally reconstructed into 3-D volumes, with advanced tools for 3D visualisation and quantification available.
Instrument:
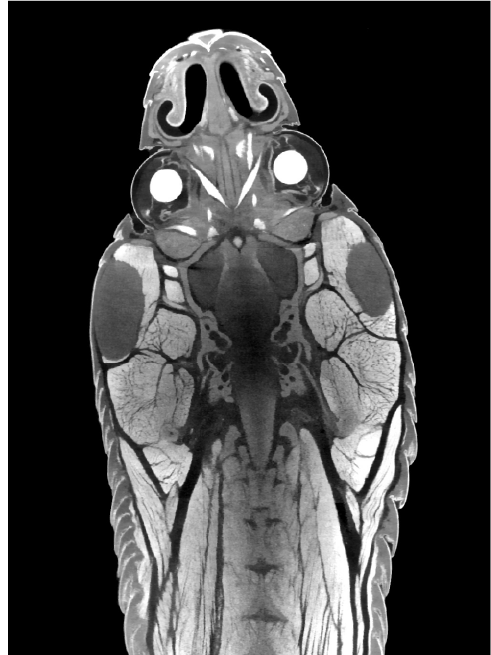
In vivo/ Ex vivo X-ray micro computed tomography (micro-CT)
The Skyscan 1176 system is a live animal X-ray microtomography system which enables high-resolution 3D imaging of anaesthetised mice or rats using low X-ray doses. The system is able to provide morphological detail of tissues including bone, muscle and fat while contrast agents can be used to produce 3D imaging of blood vessels, lymphatics and gastrointestinal spaces.
Variable X-ray applied voltages and filters provide scanning flexibility to allow imaging of a wide range of samples from lung tissue to bone with titanium implants as well as non-animal specimens. The instrument caters for full body mouse and rat scanning and for scanning of distal limbs of bigger animals such as rabbits.
An integrated physiological monitoring subsystem provides breathing and electrocardiogram gating to improve thoracic imaging.
Instrument:
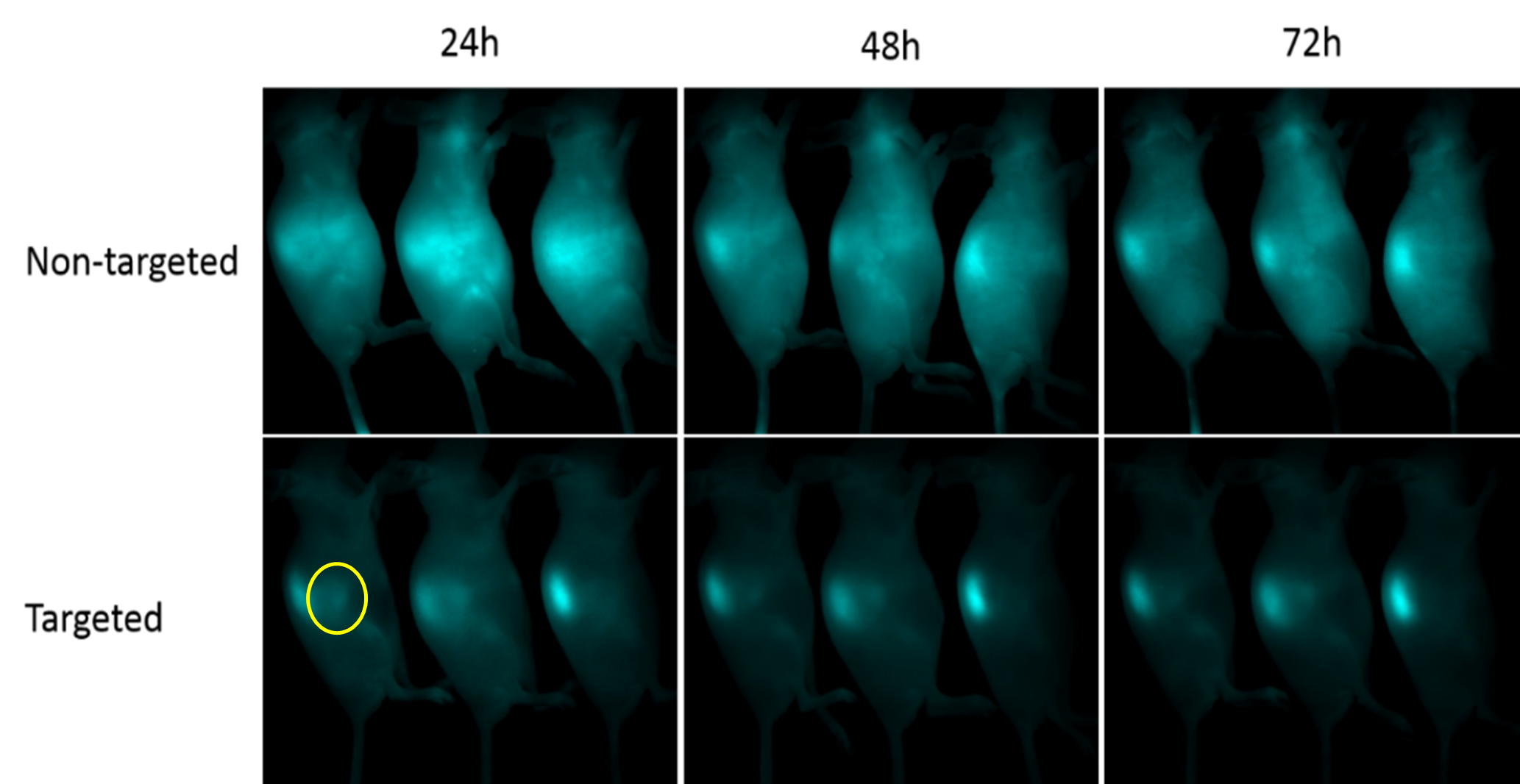
In vivo /Ex vivo multispectral fluorescence imaging and unmixing
In vivo fluorescence imaging detects the light emitted by a fluorescently tagged gene, molecule or cell in an animal and provides non-invasive analyses of the strength of the fluorescent signal. The light emitted by a fluorophore can be measured and analysed over time in the same live animal enabling tracking of gene expression, disease or tumour progression or the effects of a new drug.
More than one fluorescent signal can be imaged in vivo simultaneously. Spectral unmixing techniques can be used to unmix (separate) fluorescent signals (500-950 nm) within the same tissue as well as remove any autofluorescence.
It’s a high throughput technique capable of imaging three mice simultaneously within five minutes of acquisition time.
Instrument:
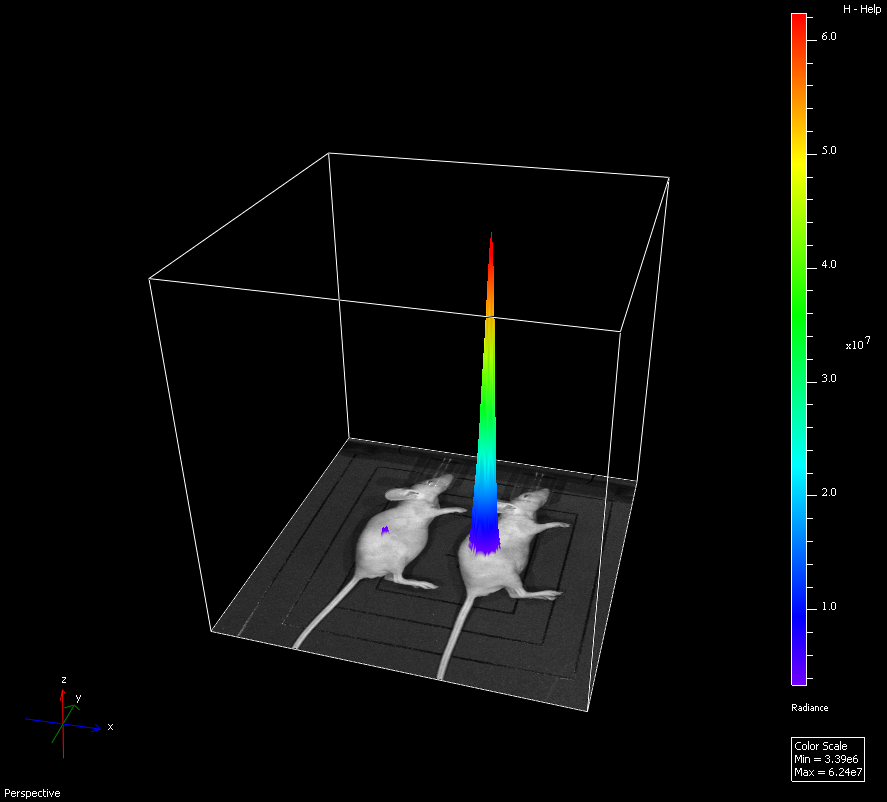
In vivo/ Ex vivo Bioluminescence imaging
In vivo bioluminescence imaging is a highly sensitive technique for measuring gene expression or for tracking cells noninvasively in a small animal. Bioluminescence is the production and emission of light by a living organism as a result of a chemical reaction between a substrate, for example luciferin and a luciferase enzyme. Cells or genes can be tagged with luciferase and imaged within an animal. Since light is endogenously emitted in response to stimulation no excitation light source is required so background light levels are extremely low and will not obscure low light signals.
Instrument:
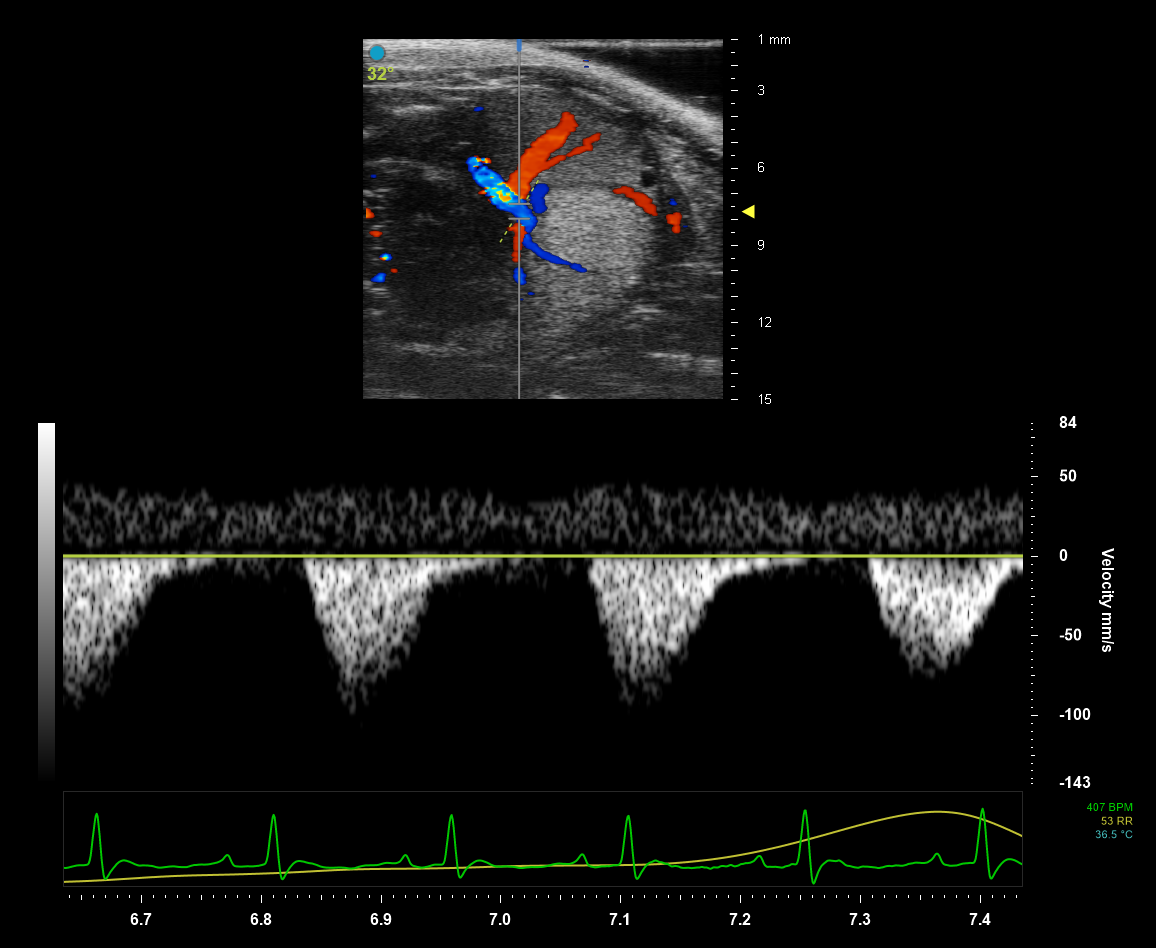
High Frequency Ultrasound and Photoacoustic Imaging
Our High Frequency Ultrasound transducers deliver an acoustic pulse into the small animal’s body. Tissues of different densities absorb and reflect sound waves differently resulting in highresolution grayscale images when the partially reflected sound waves return to the transducer. Photoacoustic imaging allows the delivery of light energy that is absorbed by tissues causing a thermoelastic expansion. This expansion then generates ultrasound waves that are detected by the transducer and produce images of optical absorption contrast within tissues. New laser technology provides faster, more sensitive image acquisition at a wider wavelength range (680 - 970 nm and 1200 - 2000 nm).
Instrument:
FAQ
-
How do I access your preclinical facilities?Please contact us to discuss your research project and its requirements. Once your project is approved and you are ready to start imaging, you can book via our online portal https://bookings.cmca.uwa.edu.au
-
What preparation is needed for my samples / subjects?Every project and instrument is different! We will discuss with you any sample or subject preparation that is required before you start your project. We can also assist with ethical approval submissions if required.
-
How long does each scan take?Scanning time will depend on the size of the sample, the type of imaging you want, the resolution, and a number of other variables. We will provide a time estimate when we plan your project with you.
-
Do I need to acknowledge WA NIF and its staff in a publication?The WA NIF and any staff that have assisted you must be formally acknowledged in any publications resulting from WA NIF usage. In some circumstances, NIF staff should be considered for authorship.
-
Will I perform the scans myself, and what supervision is available?We offer instrument training which equip our users to operate instrument by themselves! Experts are available to help plan your study, train you or your staff/students, and troubleshooting. We can set up a fee-for-service if you do not wish to be involved in the imaging process. Our academic staff can collaborate and help you with grant preparation, study design, and publication.
-
Which system is appropriate for my samples?If you are not exactly sure how best to image your samples, please contact us to discuss your options and decide what type of imaging would be ideal.
-
How do I credit WA NIF?Please include the following acknowledgement in your scientific publications, presentations and posters: “The authors acknowledge the facilities and scientific and technical assistance of the WA National Imaging Facility, a National Collaborative Research Infrastructure Strategy (NCRIS) capability, at the University of Western Australia.”
-
What analysis and results can I expect?You will receive all of the images you obtain on our equipment. For quantitative results, we can help and train you in a wide range of post-processing and analysis techniques. For on-demand analysis performed by our staff, please contact us with your query.

Location and contact
Email: [email protected]
Address: WA National Imaging Facility
Level 3, Harry Perkins Institute of Medical Research
QEII Medical Centre campus
Nedlands WA 6009
